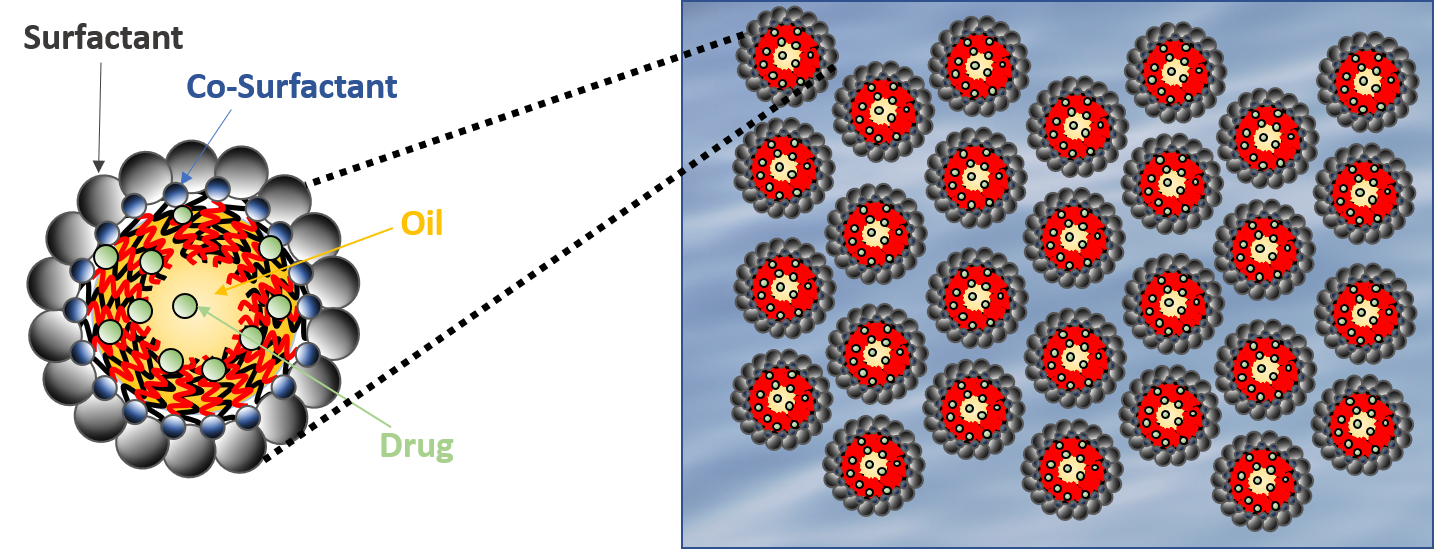
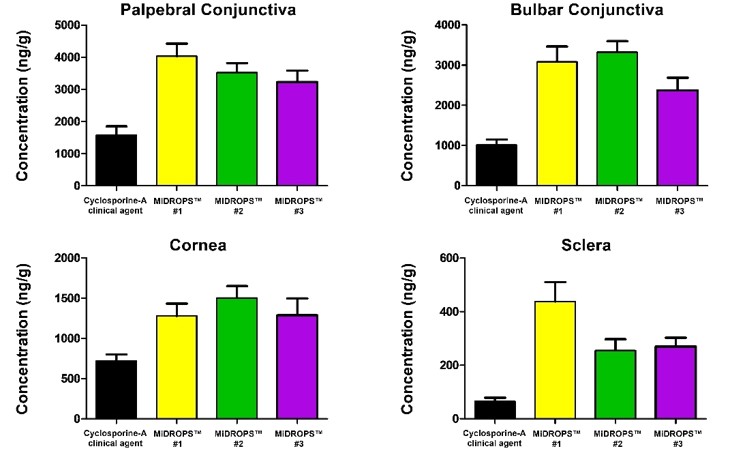
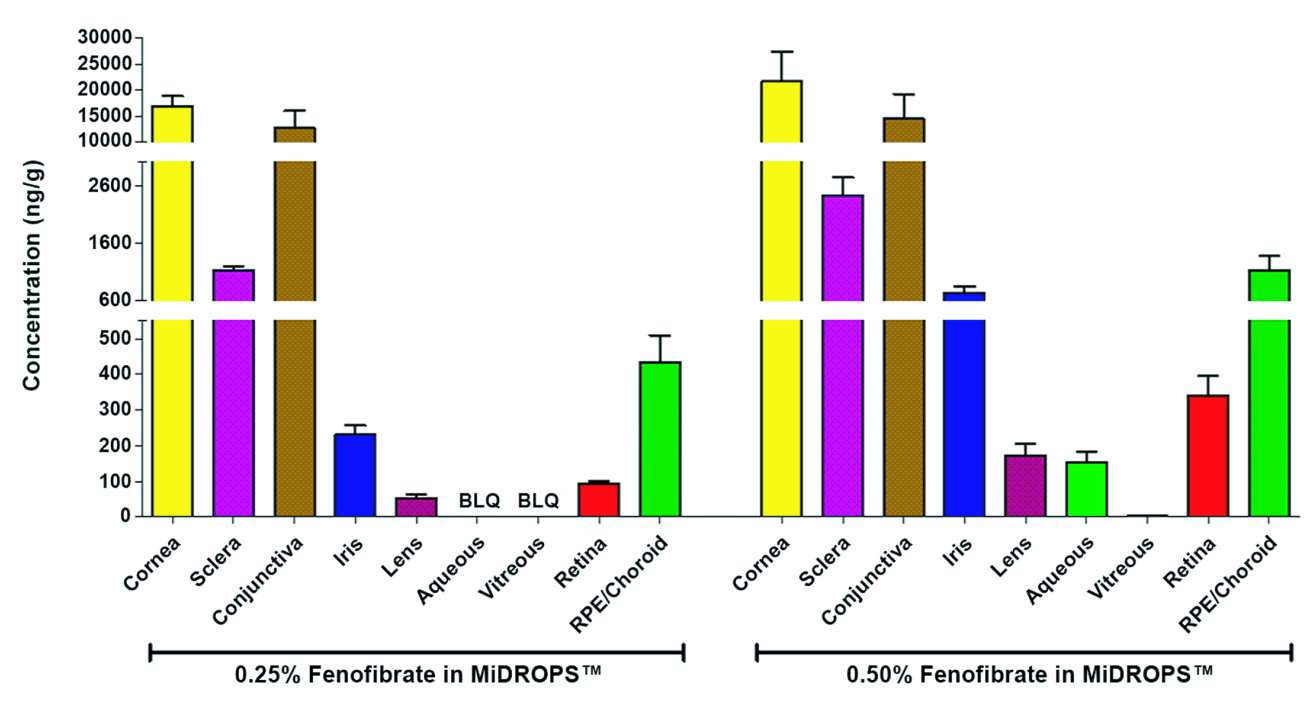
Microemulsion Drug Ocular Penetration System MiDROPS®
While eyedrop formulations have been classically used to deliver soluble molecules to the anterior segment, there is a strong desire to develop formulations which can deliver lipophilic molecules into the eye by means of a stable and comfortable eyedrop formulation. EyeCRO has developed MiDROPS®, a platform technology consisting of millions of formulations which can readily solubilize high concentrations of lipophilic agents and deliver them in abundant quantities to both the anterior segment and posterior segment. These single-phase microemulsions are self-assembling, and thus at a thermodynamic minimum which confers long term stability. Substantial proof of concept for delivery, efficacy, and safety has been demonstrated with multiple APIs in mice, rats, rabbits, and non-human primates. MiDROPS® are patent protected in all major world jurisdictions.
| Country | Award Date | Patent Number |
| Australia | 11/22/2018 | 2014244154 |
| Canada | 04/09/2019 | TBD |
| China P.R. | 09/28/2018 | ZL201480027477.7 |
| European Union | 05/23/2018 | 2968139 |
| India | 01/02/2019 | 305229 |
| Japan | 06/08/2018 | 6348567 |
| United States | 03/03/2015 | 8,968,775 |
| United States | 10/06/2015 | 9,149,453 |

Contact us to learn how MiDROPS® can help you deliver your drug with a comfortable and safe eyedrop
