Oxygen Induced Retinopathy
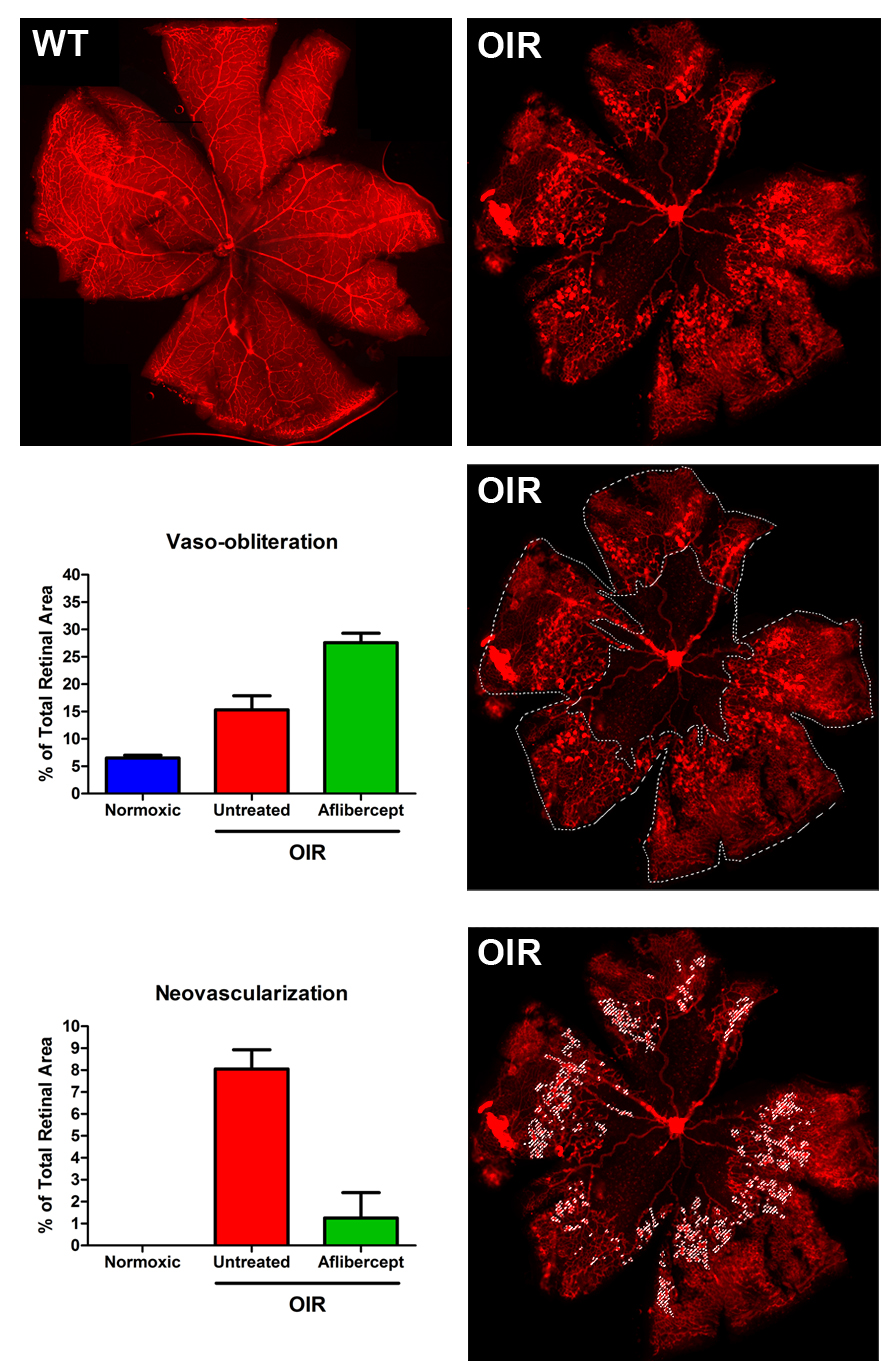
The Oxygen-induced Retinopathy (OIR) model is a useful system to study the effect of a test agent to affect ophthalmic neovascular disease that occurs in clinical diseases such as Retinopathy of Prematurity, Wet Age-Related Macular Degeneration, and Proliferative Diabetic Retinopathy. This model is an established and dependable system to evaluate therapeutics targeting vessel atrophy and regrowth, along with pathological neovascularization and tuft regression.
OIR occurs in the developing retina when neonate pups are exposed to high-oxygen levels then returned to a normal oxygen environment. In mice, newborn pups are placed in an O2- regulated chamber set to 75% O2 from postnatal day 8 to 13. Increased oxygen concentrations inhibit the production of multiple growth factors critical for vascular development, such as vascular endothelial growth factor (VEGF) and erythropoietin(1-2). Without the support of these proteins, the vessels in the retina destabilize and deteriorate, leading to vaso-obliteration around the optic nerve. During stage 2 of OIR, the pups are returned to room air (21% O2) at P13, and the avascular retina becomes hypoxic which activates transcriptional networks governed by hypoxia inducible factor (HIF) proteins. This generates substantial expression of VEGF and other growth factors to initiate a rapid, yet faulty and pathological neovascularization. The neovascular response peaks at postnatal day 18 in the form of “tufts” and then regresses between P19 and P26.
The murine OIR model has been well described and utilized by numerous groups and has proven crucial in the development of several clinical pharmaceuticals used to treat aberrant vessel formation in the retina3-6. eyecro has standardized and optimized protocols to enable efficient and meaningful pharmacology studies which generate dependable, reproducible, and quantifiable phenotypes. Multiple endpoints are available, including: 1) Vaso-obliteration; 2) Neovascular tuft formation; 3) Vessel regrowth; and 4) Tuft regression which can be quantified on retinal flat mounts at study termination using fluorescence microscopy and software analyses.
1. Chen J, Connor KM, Aderman CM, Smith LEH. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest 2008 02;118(2):526-33.
2. Smith LH, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, Schaeffer JM, Senger DR. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nature Med 1999 5;12:1390.
3. Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LEH. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nature Protocols 2009 11;4(11):1565-73.
4. Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009 Feb 26;457(7233):1103-8.
5. Hellström M, Li-Kun Phng, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson A, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe M, Kalén M, Gerhardt H. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 2007 Feb 15;445(7129):776-80.
6. Kubota Y, Hirashima M, Kishi K, Stewart CL, Suda T. Leukemia inhibitory factor regulates microvessel density by modulating oxygen-dependent VEGF expression in mice. J Clin Invest 2008 07;118(7):2393-403.
